Yoon, Park, Shin, and Ahn: Developing a core competency model for translational medicine curriculum
Abstract
Purpose
This study aimed to develop a core competency model for translational medicine curriculum in the Korean graduate education context.
Methods
We invited specialists and key stakeholders to develop a consensus on a core competency model. The working group composed of 17 specialists made an initial draft of a core competency model based on the literature review. The initial draft was sent to the survey group by email to ask whether they agreed or disagreed with each core competency. The working group simplified, merged, or excluded the competencies that received less than 80% agreement among the 43 survey respondents. The working group also reorganized the order of the domains and competencies based on the survey results, and clustered the domains into four major areas.
Results
The final core competency model has four areas, 12 domains, and 34 core competencies. The major areas are theory-based problem assessment and formulation, study design and measurement, study implementation, and literature review and critique.
Conclusion
This new core competency model will provide guidance for the competency based education of translational medicine in Korea.
Keywords: Competency-based education, Curriculum, Translational medical research
Introduction
Over the last few decades, the translation of basic scientific discoveries into clinical applications and public health improvements has become a major focus in biomedical research [ 1]. Translational medicine research fosters the multidirectional integration of basic science, clinical medicine, and population-based approach, to ultimately improve the health of the public [ 2]. The concept and the role of translational medicine research has been more clearly defined by the Clinical and Translational Science Award (CTSA) community in recent years. The CTSA Education and Career Development Key Function Committee collaborated over several years to identify 14 thematic areas of core competencies for clinical and translational medicine research [ 3]. These core competencies let the directors of translational medicine degree programs to be sure that their curriculum is comprehensive and that the students achieve these competencies through their training programs [ 4]. However, the CTSA core competencies which were developed in the context of the United States of America cannot be directly applied to other countries throughout the world due to their different system, contexts and cultural backgrounds. In the Korean academy, core competencies for translational medicine curriculum have not yet been clearly defined. Without a clear definition, developers of translational medicine curriculum will struggle to define specific program objectives, to specify the knowledge and skills that trainees are expected to develop, to select appropriate teaching and learning methods, and to assess whether competencies are achieved [ 2]. This study aimed to develop a core competency model for translational medicine curriculum in the Korean graduate education context. If we intend to develop a clear and valid model that is widely accepted within the Korean academy, a consensus should exist among the specialists and key stakeholders involved in the field of translational medicine research in Korea. This study invited specialists and key stakeholders to develop a consensus on a core competency model.
Methods
1. Working group
Seventeen specialists were invited to take part in the working group. Most of them were translational medicine specialists with medical background from the Department of Translational Medicine of a single medical college. Two other specialists who had both medical background and doctor of philosophy in education were from the Department of Medical Education at the same institution. Most of the members of the working group were also included in the survey group later.
2. Survey group
Translational medicine specialists and key stakeholders were invited to participate in the survey group to collect their ideas and arrive at a consensus on the core competencies of translational medicine research. Eighty specialists engaging in translational medicine research at a single medical college made up most of the survey group. Twenty-five other specialists and key stakeholders from different colleges, universities, and research institutions in Korea were also added to the list. They survey results were collected only from the participants who agreed with the purpose of the survey.
3. Process
First, the working group reviewed previous studies on core competencies and training programs for clinical and translational medicine research from global perspectives [ 3, 5- 7]. Then, the working group made an initial draft of the core competency model in English based on the literature review. The initial draft was sent to the survey group by email to ask whether they agreed or disagreed with each core competency. The survey group was also allowed to propose new competencies that were not listed on the initial draft. The email was sent 3 times or until a reply was received. Opinions on each domain of core competencies were also collected through the survey. After the survey, the working group simplified, merged, or excluded the competencies that received less than 80% agreement among the survey respondents. The working group also reorganized the order of the domains and competencies based on the survey results, and clustered the domains into four major areas. Finally, the working group made a list of specific competencies for each core competency that describe the core competencies in detail. The English version of the core competency model was translated into Korean by the working group.
Results
1. Initial draft and survey results
The initial draft had 13 domains and 46 core competencies. 41% (43 out of 105) of the survey group responded to the survey. Thirty-five specialists from a single medical college and eight specialists from other institutions responded to the survey on the initial draft ( Table 1). Eighty percent (36 out of 46) of the initial core competencies received an agreement from above 80% of the survey group. The average rate of agreement with the competencies was 87%±10%. The survey respondents expressed several major opinions on the initial draft of the core competency model. First, they stated that there should be an order among the domains and competencies in the model. Many of them suggested that the domains should be aligned from the fundamental elements to the practical elements, and that the competencies in each domain should be aligned from early and basic competencies to late and more difficult competencies. Second, they pointed out that some of the competencies overlapped with each other, and could be combined. Third, they indicated that some of the competencies seemed to go beyond the scope of translational medicine research and were more related to highly specialized expertise in other specific fields.
2. Core competency model
The final core competency model has four areas, 12 domains, and 34 core competencies ( Table 2). The major areas, domains, and competencies are aligned from the innermost, fundamental elements to the outermost practical elements. The working group developed a round target figure to symbolize this model ( Fig. 1). Each core competency has two to five specific competencies that describe the core competencies in detail ( Appendix 1).
Discussion
Through this study, we were able to develop a core competency model for translational medicine curriculum in graduate education based on a consensus among the related specialists and key stakeholders in Korea. In most of the previous studies, the process to reach the consensus among the stakeholders was not clearly described [ 5, 8]. This study adapted a two-step approach that was also used in a previous study, inviting the working group and a broader opinion group consecutively to reach a consensus [ 6]. The final core competency model has four areas, 12 domains, and 34 core competencies. Many of the domains and competencies in the Korean competency model are similar to those of other competency models in the literature. This may be due to the general concepts and common methodologies shared in biomedical research and the universal definition of translational medicine research worldwide [ 9]. However, there are also some differences between the Korean model and the other models. Our Korean model is structured in a more logical and systematic way. The domains are categorized into four major areas, and the major areas, domains, and competencies are aligned from the fundamental and basic elements to the practical elements. The Korean competency model also places a greater emphasis on the basic concepts and principles of medical science than other models. This may reflect the research environment in Korea, in which most translational medicine research is conducted by doctor of medicine scientists in medical institutions. Finally, there are two to five specific competencies for each core competency that describe the core competencies in detail, and the whole model is described in both English and Korean. This will help the Korean researchers and program directors understand and utilize this model. Developing and evaluating competence within the translational medicine research field is not only important for educational needs, but also for the accountability of this field [ 9]. Core competencies for translational medicine research define the knowledge, skills, and attitudes needed to function successfully within this field [ 10, 11]. Achieving competence is a developmental process that requires a gradual progression toward the integration of these knowledge, skills, and attitudes [ 12]. Competency-based education is a learning paradigm focused on what learners need to know and be able to do, based on the goals and objectives of the curriculum [ 13, 14]. Thus, this new core competency model could provide guidance for the education and training of translational medicine researchers in Korea. This study also has some limitations. We were not able to invite all the specialists and stakeholders involved in the field of translational medicine research in Korea. Most members of the working group were from a single institution, and the majority of the respondents to the survey were also from the same institution. Some of the members in the working group also participated in the survey. The last version of the core competency model was not sent to the survey group again for an eventual agreement. Thus, this core competency model should not be perceived as a final fixed version but rather as an initial version that could be revised and updated continuously. Moreover, any individual institution may adapt and modify this model according to its specific needs and circumstances.
We developed a core competency model for translational medicine curriculum in Korea through a consensus among the specialists and key stakeholders in the field. This core competency model will help the developers of translational research education and training programs to articulate specific program objectives, create an appropriate curriculum, and assess whether program objectives and competency requirements are met.
Fig. 1.
The Major Four Areas and 12 Domains of the Core Competency Model
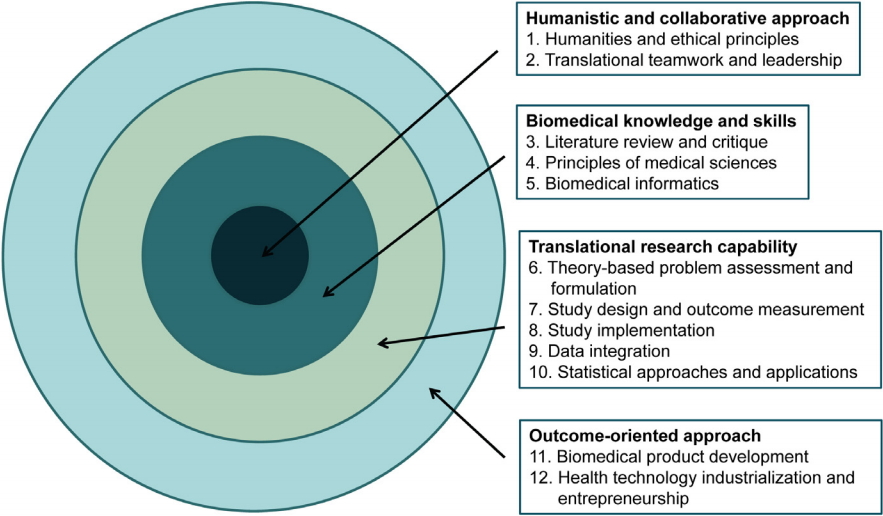
Table 1.
Survey Results Regarding the Initial Draft of the Core Competency Model
|
Domains |
Core competencies |
Agreement (%) |
|
1.Theory-based problem assessment and formulation |
1. Identify major clinical/public health problems and relevant translational research questions |
100 |
|
2. Formulate sound hypotheses |
95 |
|
3. Develop innovative, testable clinical and translational research questions |
93 |
|
4. Extract information from the scientific literature that yields scientific insight for research innovation |
95 |
|
2.Study design and measurement |
1. Formulate a well-defined clinical or translational research question to be studied in human or animal models |
88 |
|
2. Propose study designs for addressing a clinical or translational research question, and assess the strengths and weaknesses of possible study designs for a given clinical or translational research question |
93 |
|
3. Design appropriate, ethically sound, and hypothesis-driven clinical studies |
95 |
|
4. Identify important outcome measures for incorporation into patient-oriented clinical trial design |
93 |
|
5. Manage research and development projects |
84 |
|
3.Study implementation |
1. Compare the feasibility, efficiency, and ability to derive unbiased inferences from different translational research study designs |
95 |
|
2. Assess threats to internal validity in any planned or completed translational study |
93 |
|
3. Incorporate regulatory precepts into the design of any translational study |
86 |
|
4.Literature review and critique |
1. Describe the current state of knowledge about a biomedical, clinical, or public health problem |
86 |
|
2. Use evidence as the basis of the critique and interpretation of results of published studies |
95 |
|
3. Identify potential sources of bias and variation in published studies |
93 |
|
5.Data integration |
1. Assess data sources and data quality to answer specific clinical or translational research questions |
95 |
|
2. Implement quality assurance and control procedures for different study designs and analysis |
93 |
|
3. Define bias in clinical and translational research |
91 |
|
6.Statistical approaches and applications |
1. Build effective statistical expertise into research teams |
95 |
|
2. Determine and justify the appropriate statistical technique(s) for a specific research question and concomitant study design |
100 |
|
7.Health informatics |
1. Identify modern information systems for collecting, organizing, managing, and accessing clinical data |
95 |
|
2. Utilize best practices in informatics for the organization and management of biomedical/health information |
86 |
|
8.Preclinical drug development |
1. Define drug development goals, and explain the overall process of drug development |
84 |
|
2. Propose preclinical study designs for lead optimization, formulation developments, pharmacology studies, and toxicity studies |
67 |
|
3. Describe investigational new drug profiling, new drug application, and the requirements of theFood and Drug Administration for drug and biologic products |
81 |
|
9.Principles of medical sciences |
1. Understand the core concepts of anatomy and physiology of body systems, and pathogenesis and treatment (non-MD students) |
93 |
|
2. Understand the core concepts of basic science (MD students) |
95 |
|
10.Scientific communication |
1. Communicate scientific findings to your peers and to disseminate scientific knowledge, through medical writing, presentations, and publications |
98 |
|
2. Assess the clinical implications of scientific information |
84 |
|
11.Translational teamwork and leadership |
1. Build an interdisciplinary/intradisciplinary/multidisciplinary team that matches the objectives of the research problem |
86 |
|
2. Manage an interdisciplinary team of scientists |
72 |
|
3. Demonstrate group decision-making techniques |
67 |
|
4. Participate in cross-disciplinary training and mentoring |
81 |
|
12.Health technology industrialization and entrepreneurship |
1. Explain the utility and mechanism of commercialization for clinical and translational research findings, the patent process, and technology transfer |
74 |
|
2. Transform translational idea into a high-impact venture |
63 |
|
3.Develop the effective applications for funding from appropriate governmental and/or non-governmental sources at each stage of translating research to clinical care of patients |
72 |
|
4. Propose technology-inspired models for success, and be able to manage financial and funding issues |
70 |
|
5. Experience at least one part of the industrialization process of translational medical research products such as pharmaceuticals, medical devices, or biologics |
77 |
|
13.Humanities and ethical principles |
1. Identify the historical issues of translational medical research |
86 |
|
2. Understand demographic, cultural, geographic, and ethnic differences inhealth care and aim for globalization |
81 |
|
3. Propose multi-disciplinary collaborations and consilience in translational medicine |
79 |
|
4. Demonstrate the knowledge of research ethicsfrom basic to clinical studies |
88 |
|
5. Describe the requirements and processes of Food and Drug Administration approval for medicine, medical supplies and devices |
84 |
|
6. Apply the principles and requirements of institutional review board approval |
100 |
|
7. Explain conflict of interest management in research |
91 |
|
8. Apply the principles of conflictsof interest accompanying research and presentations |
86 |
|
Average rate of agreement |
|
87 |
Table 2.
Core Competency Model for Translational Medicine Research in Korea
|
Areas |
Domains |
Core competencies |
|
Humanistic and collaborative approach |
1.Humanities and ethical principles |
1. Develop humanities-based consilience in translational medicine |
|
2. Demonstrate the knowledge of research ethics from basic to clinical studies |
|
3. Apply the principles of conflicts of interest accompanying research and presentations |
|
4. Apply the principles and requirements of institutional review board approval |
|
2.Translational teamwork and leadership |
1. Communicate scientific findings to your peers and to disseminate scientific knowledge, through medical writing, presentations, and publications |
|
2. Build and manage an interdisciplinary/intradisciplinary/multidisciplinary team that matches the objectives of the research problem |
|
3. Participate in cross-disciplinary training and mentoring |
|
Biomedical knowledge and skills |
3.Literature review and critique |
1. Describe the current state of knowledge about a biomedical, clinical, or public health problem |
|
2. Use evidence as the basis of the critique and interpretation of results of published studies |
|
3. Identify potential sources of bias and variations in published studies |
|
4.Principles of medical sciences |
1. Understand the core concepts of anatomy and physiology of body systems, and pathogenesis and treatment |
|
2. Understand the core concepts of basic science and biomedical engineering |
|
5.Biomedical informatics |
1. Identify modern information systems for collecting, organizing, managing, and accessing clinical data |
|
2. Utilize best practices in informatics for the organization and management of biomedical information |
|
Translational research capability |
6.Theory-based problem assessment and formulation |
1. Identify major clinical/public health problems and relevant translational research questions |
|
2. Develop innovative, testable clinical and translational research questions |
|
3. Formulate sound hypotheses |
|
7.Study design and outcome measurement |
2. Assess the strengths and weaknesses of possible study designs for a given clinical or translational research question |
|
3. Design appropriate, ethically sound, and hypothesis-driven clinical studies |
|
4. Identify important outcome measures for incorporation into patient-oriented clinical trial design |
|
8.Study implementation |
1. Compare the feasibility, efficiency, and ability to derive unbiased inferences from different translational research study designs |
|
2. Assess threats to internal validity in any planned or completed translational study |
|
3. Incorporate regulatory precepts into the design of any translational study |
|
9.Data integration |
1. Assess data sources and data quality to answer specific clinical or translational research questions |
|
2. Implement quality assurance and control procedures for different study designs and analyses |
|
3. Assess potential bias and variation of source data |
|
10. Statistical approaches and applications |
1. Build effective statistical expertise into research teams |
|
2. Determine and justify the appropriate statistical techniques for a specific research question and concomitant study design |
|
Outcome-oriented approach |
11. Biomedical product development |
1. Define biomedical product development goals, and explain the overall developmental process |
|
2. Propose preclinical study designs for biomedical product development |
|
3. Describe investigational new drugprofiling, new biomedical product application, and the requirements of regulatory authorities for biomedical products |
|
12. Health technology industrialization and entrepreneurship |
1. Explain the utility and mechanism of commercialization for clinical and translational research findings, the patent process, and technology transfer |
|
2. Experience at least one part of the industrialization process of translational biomedical products |
|
3. Develop effective applications for funding from appropriate governmental and/or nongovernmental sources at each stage of translational research |
References
1. Drolet BC, Lorenzi NM. Translational research: understanding the continuum from bench to bedside. Transl Res 2011;157(1):1-5.   5. Sonstein SA, Seltzer J, Li R, Silva H, Jones CT, Daemen E. Moving from compliance to competency: a harmonized core competency framework for the clinical research professional. J Clin Res Best Pract 2014;28(3):17-23.  8. Woolf SH. The meaning of translational research and why it matters. JAMA 2008;299(2):211-213.   10. Lichtenberg JW, Portnoy SM, Bebeau MJ, et al. Challenges to the assessment of competence and competencies. Prof Psychol Res Pract 2007;38(5):474-478.  11. Lee YB. The role of the concept of competence in Korean outcome-based medical education. Korean Med Educ Rev 2013;15(3):144-150.
12. Zane TW. Domain definition: the foundation of competency assessment. Assess Update 2008;20(1):3-4.
14. Ahn JH, Yang EB. An outcome-based approach in medical curriculum development. Korean Med Educ Rev 2013;15(1):9-18.
|
|













